Content Hub:
Post-Approval Adjustments to Analytical Methods

Pharmaceutical companies frequently make post-approval adjustments to drugs due to evolving safety data, manufacturing changes, regulatory updates, or market needs. These include reformulating products, updating labelling, modifying dosage forms, or changing manufacturing processes. Such changes are governed by regulatory frameworks and require careful documentation and validation.
Among the most critical adjustments are those involving validated analytical methods. As formulations or processes evolve, analytical techniques and methods must be reassessed to ensure continued accuracy, precision, and compliance.
This hub explores how and why adjustments are made, and the regulatory expectations surrounding them.
What is Method Adjustment?
Method adjustment refers to the process of modifying validated analytical methods to accommodate changes in laboratory conditions, instrumentation, regulatory requirements or material changes.
Common situations requiring method adjustment include:
- Lab transfers - e.g., between sites or CROs
- System migrations - e.g., from HPLC to UHPLC
- Implementation of compendial methods - e.g., USP updates
- Raw material changes - e.g., new suppliers or grades
- Modernization efforts - e.g., replacing obsolete columns or hardware
Regulatory Requirements for Adjusting Analytical Methods
Whether using a method developed in-house or adapted from a pharmacopeia, understanding the regulatory framework for changes is essential. Knowing where you have flexibility—and where caution is required—can save time, avoid compliance issues, and streamline regulatory interactions.
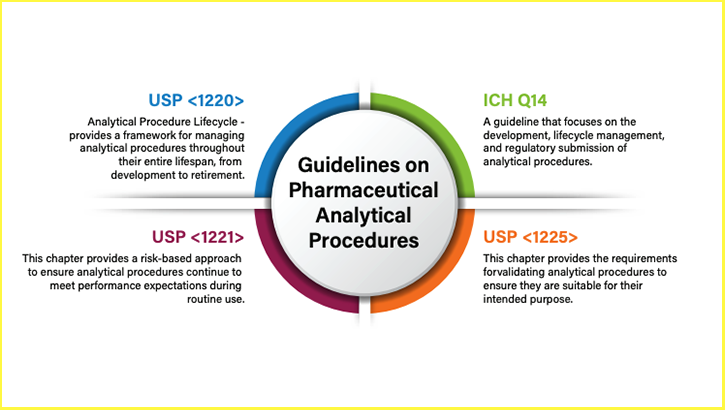
For in-house methods, ICH guidelines provide the foundation. ICH Q14 promotes a risk-based approach to development and adjustment, centered on the concept of a design space—a predefined range of parameters (e.g., flow rate, temperature) that can be modified without triggering full revalidation. Established Conditions (ECs) identify critical parameters that must remain fixed to ensure consistent performance, enabling controlled adjustments for equipment updates, material changes, or evolving lab needs. ICH Q2(R2) defines validation requirements for attributes like accuracy, precision, and specificity, while ICH Q12 offers tools such as the Post-Approval Change Management Protocol (PACMP) to manage changes efficiently.
For compendial methods in USP, EP, or JP, chapters like USP <621> allow certain adjustments (including flow rate, mobile phase pH, column dimensions and temperature) within set limits, provided system suitability is maintained. Exceeding these allowances typically requires full revalidation as for a new method.
Modern frameworks like ICH Q14 support scientifically justified flexibility, but successful changes depend on controlling variability from, e.g., instrumentation, sample prep, or the environment. Robust method design, thorough risk assessment, and lifecycle management help understand and control sources of variability, justify adjustments, maintain compliance, and protect product quality.
When and Why Analytical Methods Need Post-Approval Changes
Method Adjustments During Transfer and Migration
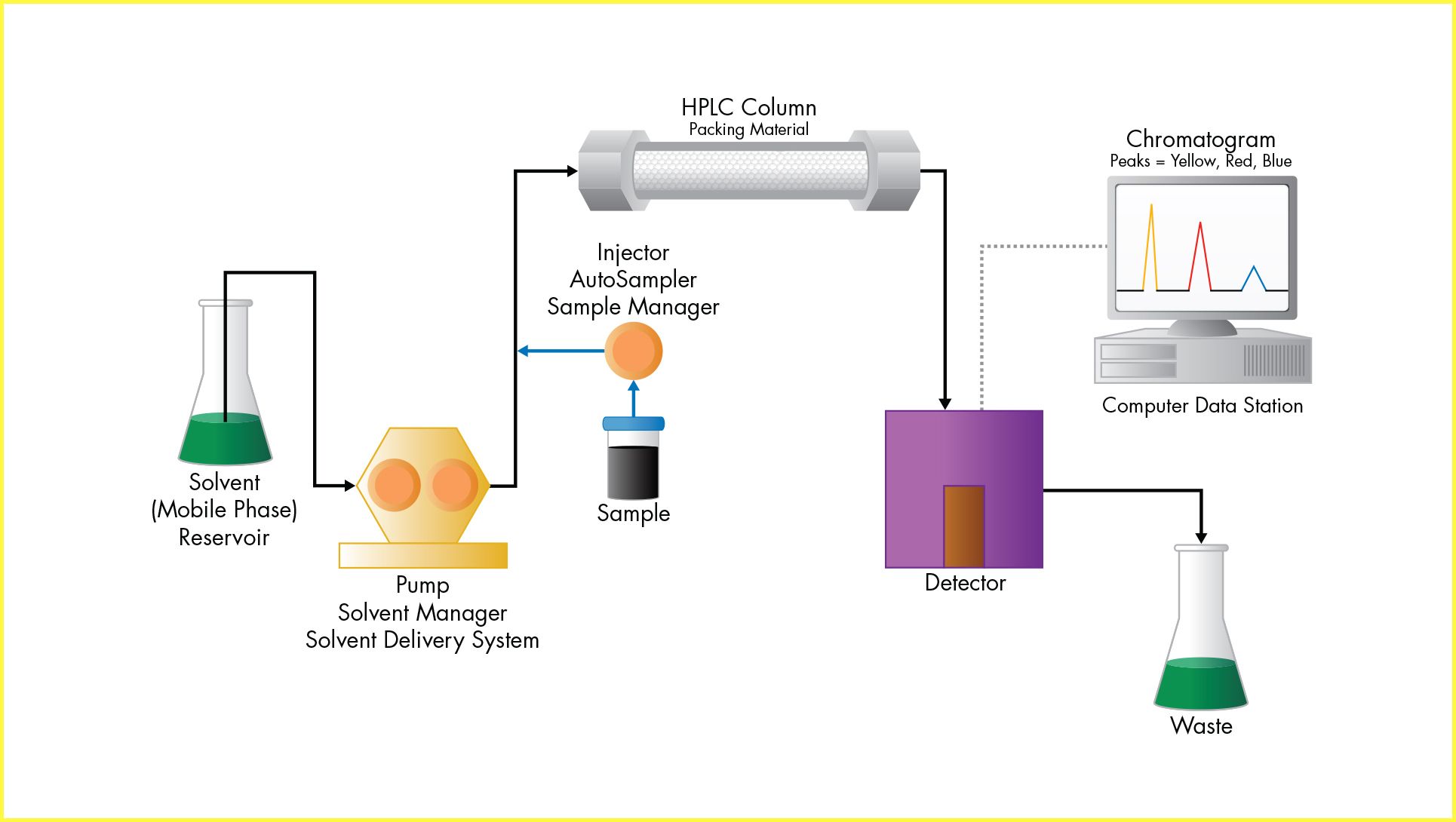
Method transfer refers to the formal movement of a validated analytical procedure from one laboratory to another, such as from R&D to QC or to external partners. Post-approval, this process must ensure the method performs equivalently in the receiving lab. Considerations include system compatibility, analyst training, and environmental conditions.
Method migration involves adapting a validated method for use on different instruments within the same lab or organization, often due to modernization or standardization efforts. When migrating methods across different platforms, which may have different specifications, adjustments may be needed to accommodate differences in dwell volume, pressure limits, and detector response. When migrating methods across different LC platforms, differences in system design, particularly system design and system volume, must be carefully considered.
Critical Factor # 1: System Design
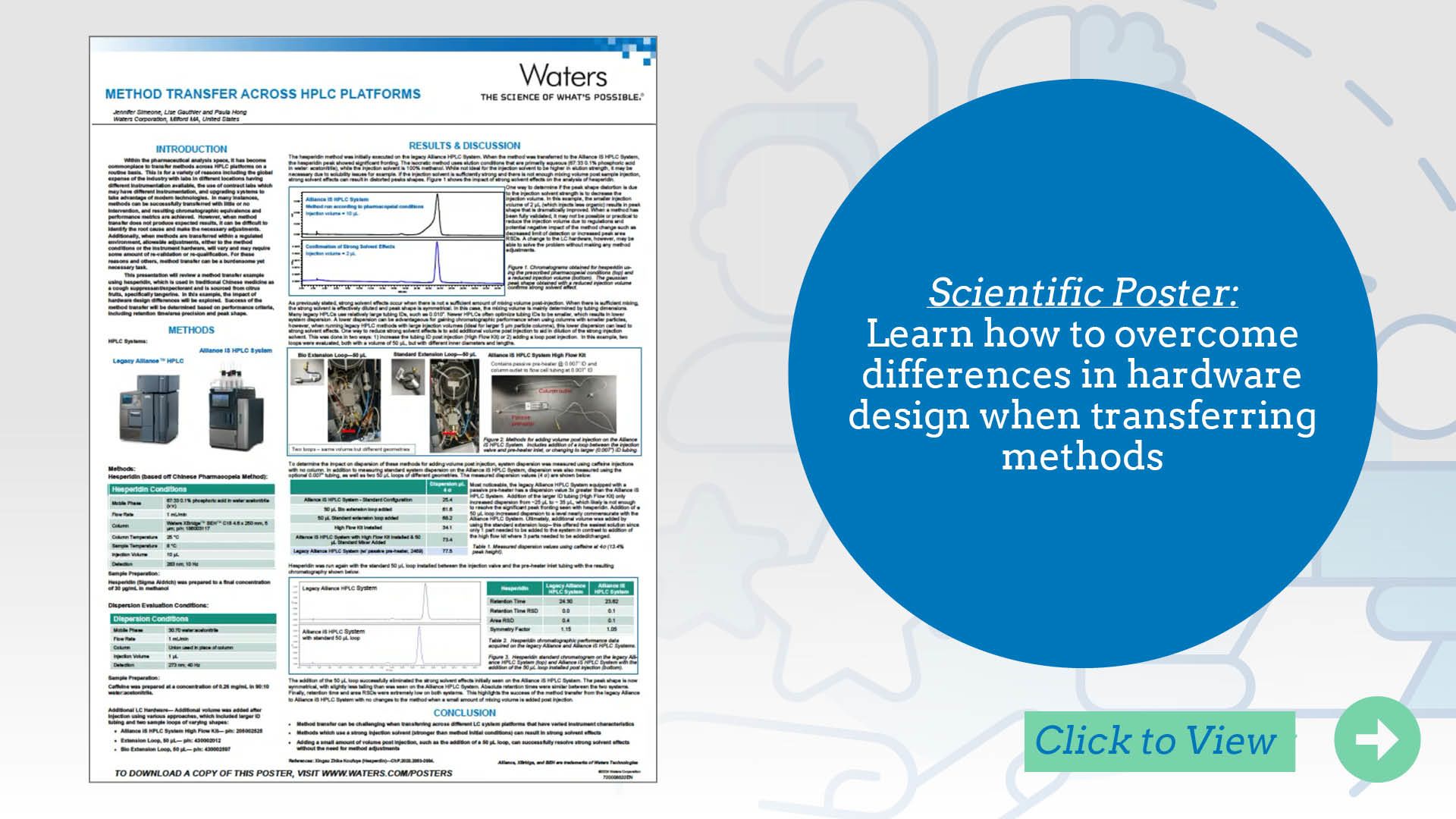
Transferring a method between LC platforms may require system-specific adjustments to maintain equivalent performance. Each element of the system design, including pump type, mixer volume, tubing dimensions, column preheater, and detector setup, directly influences chromatographic outcomes such as retention time and peak shape. Differences between platforms, especially across vendors or instrument generations, may necessitate method adjustments to ensure reproducibility.
This poster shows how mismatched tubing dimensions can cause strong solvent effects that degrade peak shape and reproducibility, and outlines strategies to mitigate them, such as increasing post-injection tubing ID or adding an extension loop on the receiving system. These steps help align the system configuration with the original platform. Accounting for such design variables is critical for successful method transfer while preserving method integrity and analytical performance.
Critical Factor #2: System Volume
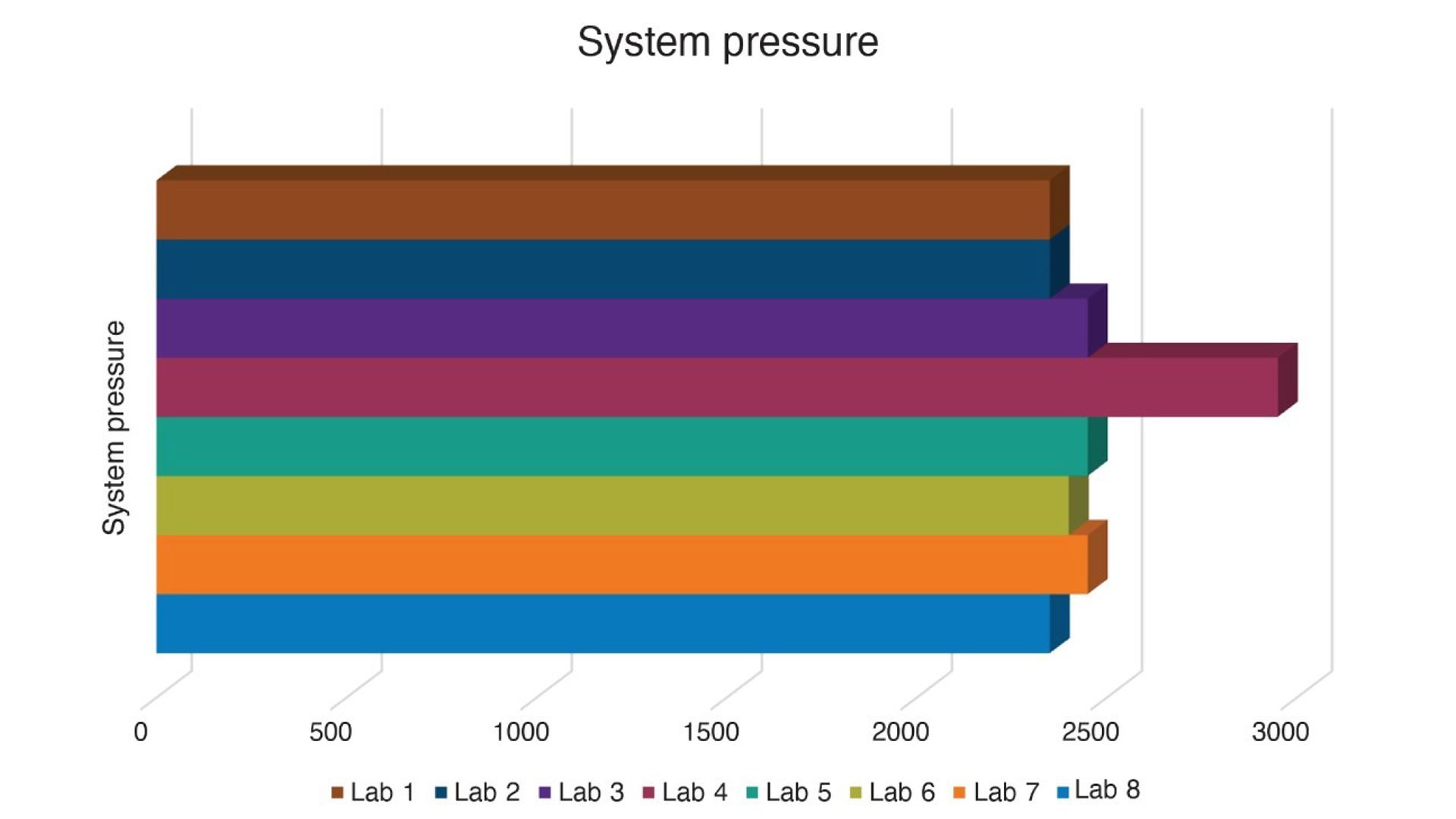
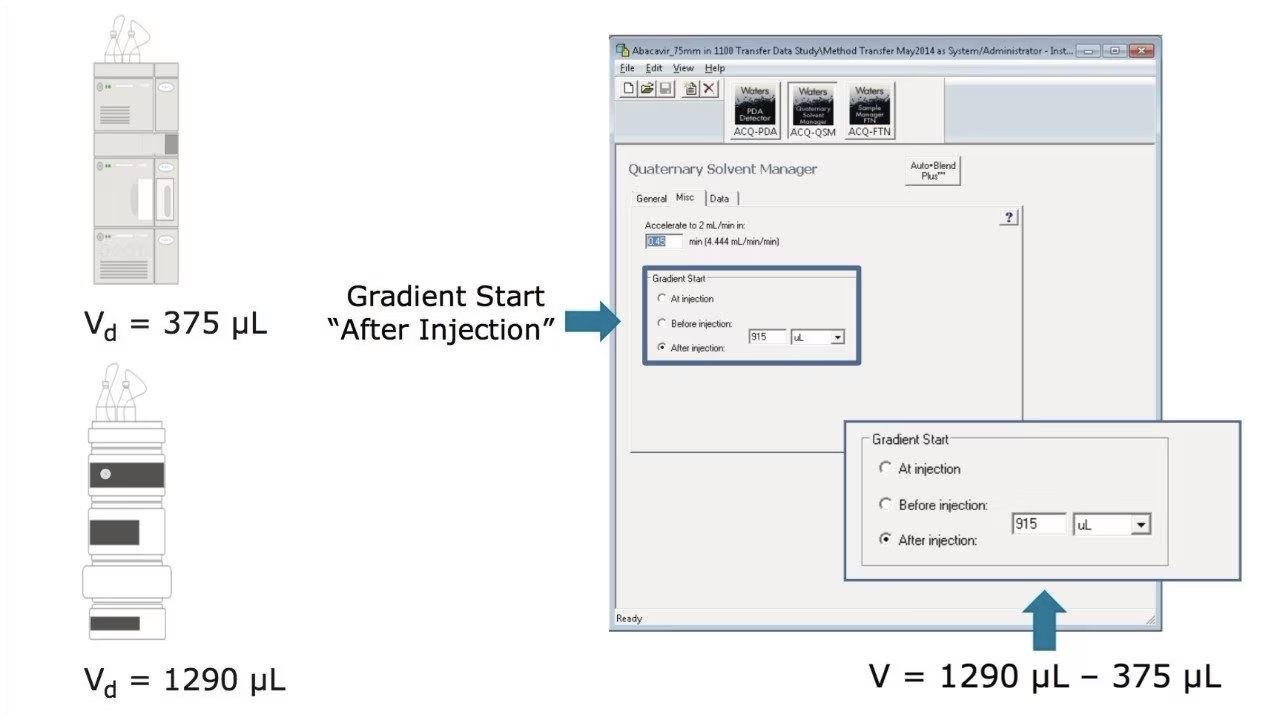
When transferring a method between LC systems, understanding and managing system volume is essential to preserving chromatographic performance. Two critical aspects of system volume, dwell volume and extra-column volume, can significantly influence retention times, peak shapes, and resolution.
Dwell volume refers to the volume between the point where the gradient is formed and the column inlet. Variations in dwell volume between systems can shift gradient delivery timing, leading to changes in retention times and potentially impacting method reproducibility. Matching dwell volumes or adjusting gradient delay times is often necessary to ensure consistent results across platforms.
Extra-column volume, on the other hand, includes all the volume outside the column that the sample travels through, such as tubing, connectors, and detector flow cells. Excessive extra-column volume can cause peak broadening and reduced resolution, particularly in high-efficiency methods or when using narrow-bore columns.
This application note explores these concepts in detail. It provides guidance on how to compensate for differences in system volume and demonstrates the impact of extra-column volume on both isocratic and gradient separations. By addressing these variables, analysts can preserve the integrity and performance of the original method across different LC platforms.
Interested in seeing more examples of successful method migration and transfer?
Explore these additional resources for practical insights and case studies
Application Note
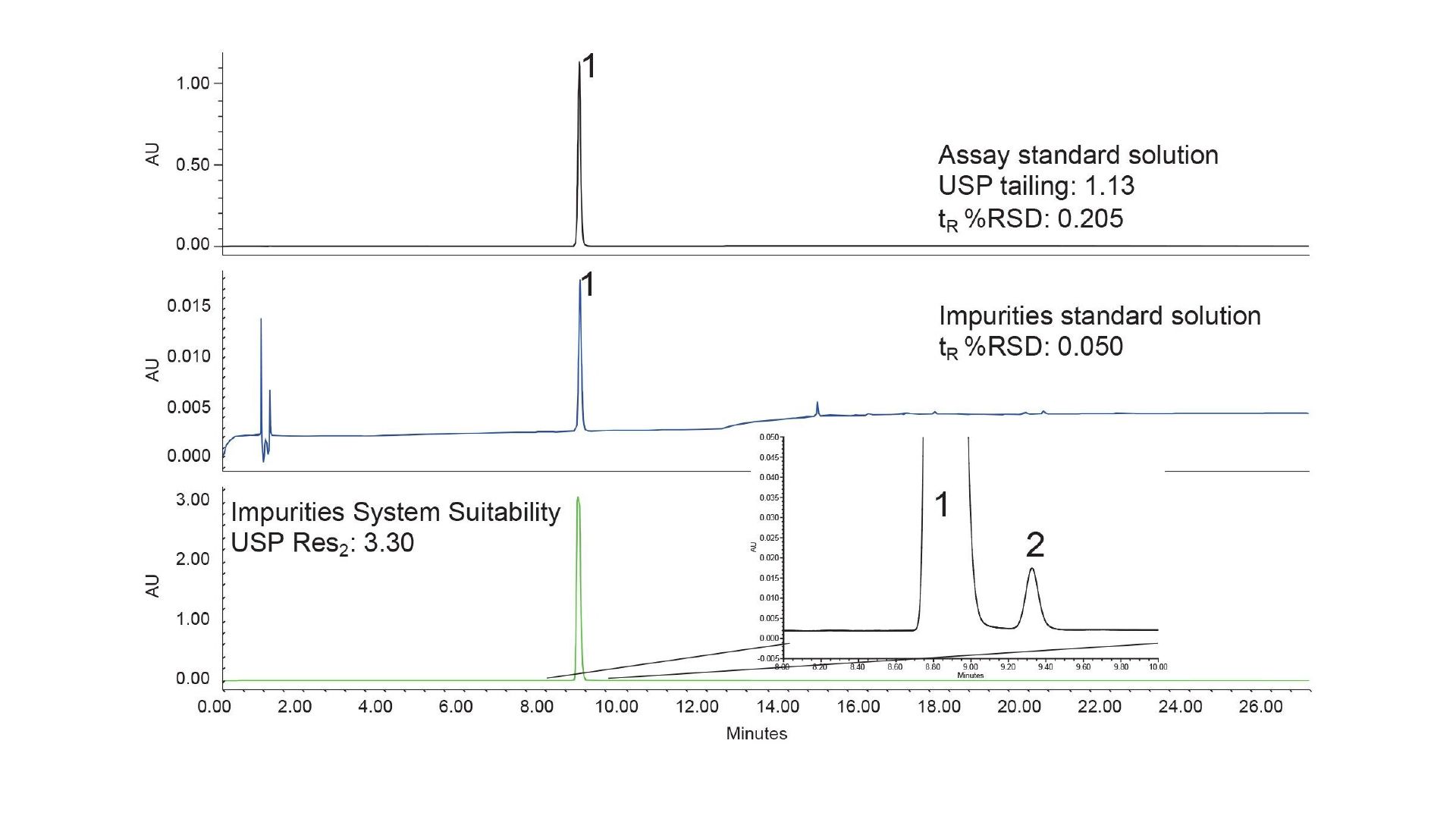
Successful Global Cross Lab Method Transfer of a USP Organic Impurities Method to an Arc HPLC Using a Risk-based Approach
Case Study
Investigating Unexpected Results of a Global Cross-Laboratory Study of a USP Organic Impurities Method on an Arc HPLC System
White Paper
Mitigating Risk of Validated Analytical Procedure Failures When Upgrading or Replacing LC Assets
Application Note
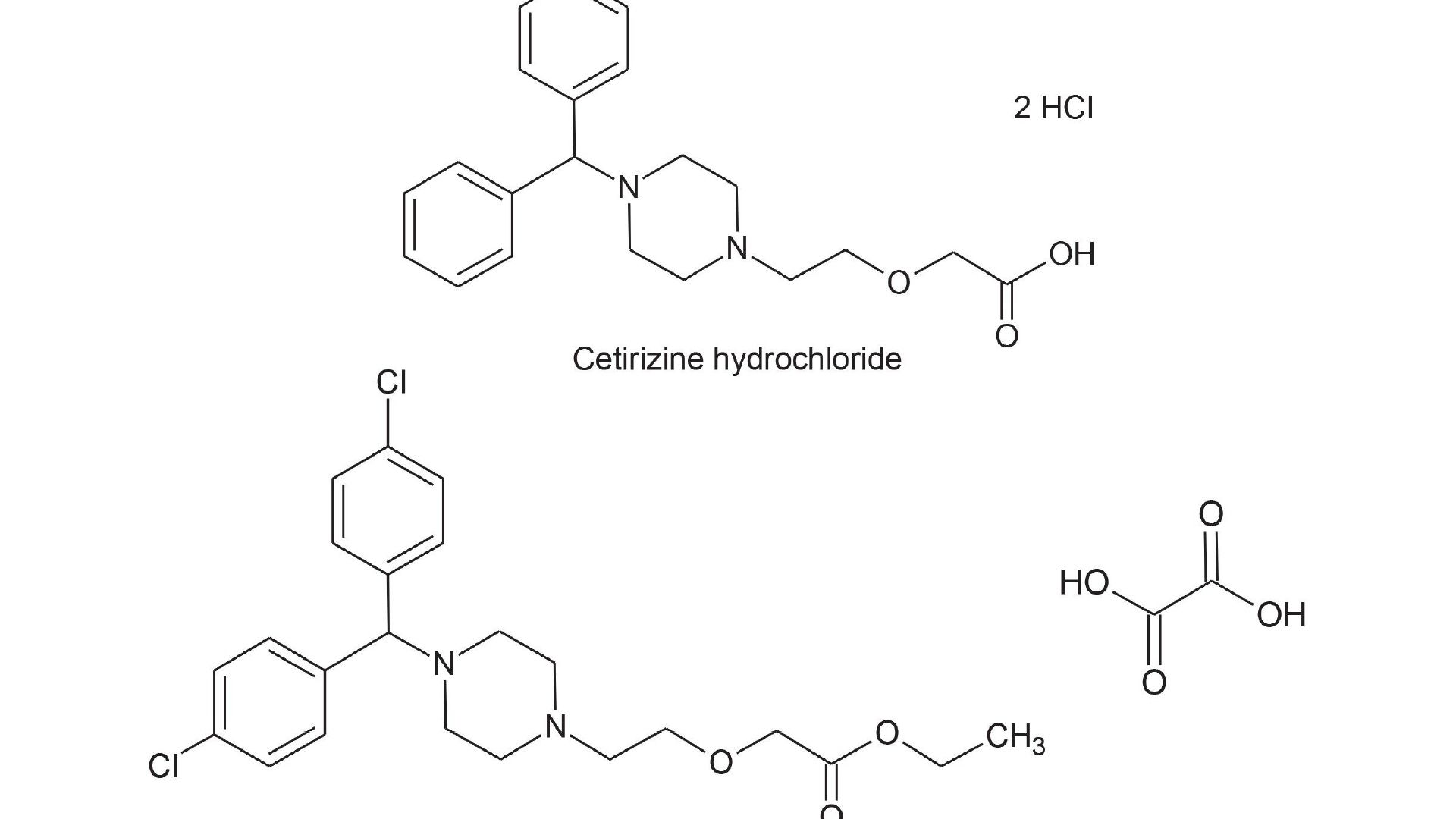
Hydrophilic Interaction Liquid Chromatography (HILIC) Method Migration Part 2: Troubleshooting Peak Splitting of Cetirizine
Implementation of compendial methods
Compendial methods, such as those published in the United States Pharmacopeia (USP), European Pharmacopoeia (EP), or Japanese Pharmacopoeia (JP), are widely used for product release and quality control. These methods are considered validated by virtue of their inclusion in the pharmacopoeia, but that does not mean they can be implemented without verification or possible adjustment.
Both FDA 21 CFR 211.194(a)(2) and EU GMP Chapter 6 make it clear: If you adopt a method that you did not originally validate, you must verify that it is appropriate for your intended use under actual conditions of use. This verification step ensures the method delivers the required accuracy, precision, and specificity in your lab, using your instruments, columns, reagents, and personnel.
Why Adjustments Are Sometimes Needed
Even with detailed compendial instructions, there are situations where method adjustments may be required:
- Instrument differences (different HPLC/ UHPLC that were used in the compendial methods vs what you plan to use)
- Column availability (different manufacturer, particle size, or dimensions within pharmacopeial allowances)
- Environmental factors (lab temperature and humidity affecting retention times or peak shape)
- Reagent variability (different lots or suppliers of mobile phase components)
When such changes are needed, compendial chapters like USP <621> Chromatography provide explicit allowances, such as:
- Adjusting column length or particle size, per constant L/dp constant between -25% to +50%
- Adjusting column internal diameter
- Varying flow rate
Staying within these ranges is considered an adjustment, not a method change, and does not require full revalidation, provided the method still meets system suitability requirements. If adjustments exceed compendial limits, the method is no longer considered compendial. At that point, it must be validated as a new method and, depending on the product, regulatory authorities may need to be notified.
A Proactive Approach to Address Excipient and Material Variability
Changes in raw materials or excipients—such as differences in grade, supplier, or composition—can significantly influence the performance of a finished drug product or its substance. Variations may affect critical attributes like solubility, assay accuracy, or impurity separation, potentially disrupting validated analytical methods. To accommodate such variability, methods may require adjustment or complete redevelopment.
In line with ICH Q14 and the design‑space paradigm, applying Analytical Quality by Design (AQbD) during method development builds flexibility from the start. Defining an Analytical Target Profile (ATP), conducting risk assessments, performing fit-for purpose experiments, and establishing a Method Operable Design Region (MODR) enable the controlled adjustment of method parameters within a validated space—variations within the MODR are not considered changes and thus don't require revalidation.
We have used an AQbD-based approach to develop a method for cold-and-cough formulations, using multiple formulations and spiking in high levels of excipients helps ensure robustness across a range of product profiles. By systematically evaluating factors like stationary phase, pH, gradient time, and temperature, we were able to define a Method Operable Design Region (MODR) that maintained consistent performance even with formulation variability. This upfront approach builds flexibility into the method, supporting post-approval changes.
Modernization efforts
Modernizing analytical workflows often involves updating methods to take advantage of improved technology. In many laboratories, this means replacing outdated or unavailable legacy columns or adapting methods to work with smaller particle sizes for faster, higher-resolution separations. While these changes can offer clear performance benefits, they must be managed carefully to preserve method integrity and ensure compliance.
When adjusting a method as part of modernization, the primary goal is to maintain its intended purpose while leveraging the capabilities of new technology. Similar to the changes to system components as mentioned above, changes to columns, such as column dimensions and particle size, may affect critical method parameters like retention time, resolution, and peak shape. Adjustments made without proper evaluation can lead to variability, compromised data quality, or regulatory concerns.
Regulatory guidelines, including ICH Q14 and pharmacopeial chapters like USP <621>, allow for certain adjustments within defined limits, provided that method performance is verified through system suitability testing. These application notes demonstrate how method modernization can be applied to significantly reduce analysis time and solvent usage while maintaining performance and regulatory compliance.
Application Notes
App Note
Improving USP Monograph Analysis Time Through Scaling by N on an HPLC System Using CORTECS™ Premier Columns
App Note
Achieving Method Modernization with the New Liquid Chromatographic Gradient Method Allowances Provided by USP General Chapter <621> Chromatography and the Arc™ HPLC System
App Note
Achieving Method Modernization with the New Liquid Chromatographic Gradient Allowances Provided by USP General Chapter <621> Chromatography and the Alliance™ iS HPLC System
App Note
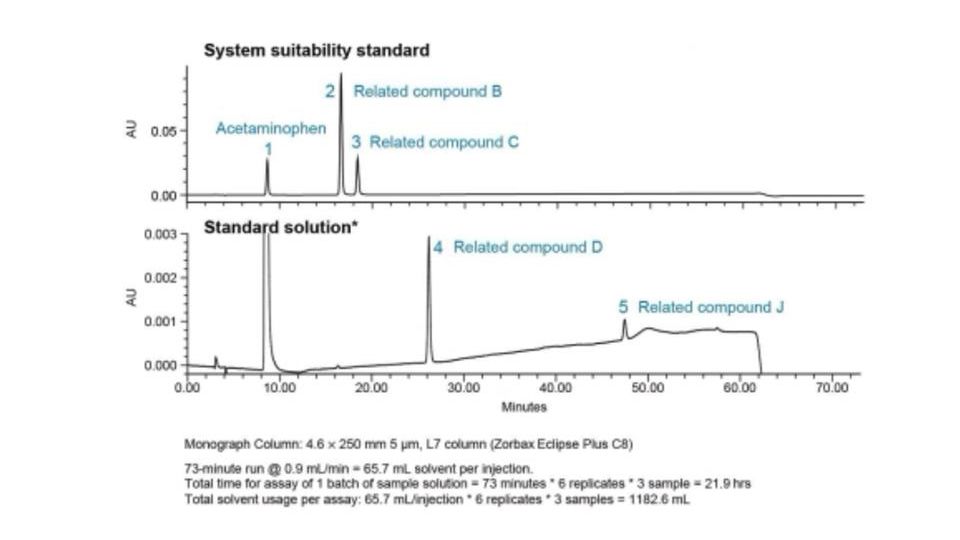
Modernization of the Acetaminophen USP Monograph Gradient HPLC Method for Impurities using USP <621> Guidelines and MaxPeak™ Premier HPS Technology
At Waters we’re set up to support you
Waters LC systems, columns, and Empower software form a complete, integrated solution designed to help labs respond quickly when analytical methods need to be adapted—whether fine-tuning existing approaches or implementing those received from another source.
- Waters LC systems deliver precise, robust, and reproducible performance and the flexibility to adapt to changing analytical requirements, without compromising data quality.
- Waters columns are engineered for scalability, with matched chemistries and dimensions that make it straightforward to adjust methods for different throughput or scale requirements, all while maintaining consistency and minimizing re-validation.
- Empower software brings it all together, offering powerful yet intuitive tools to modify, transfer, and verify methods, with secure data handling, audit trails, and version control to document every change.
- Waters’ global network of analytical services and technical experts provides essential guidance and troubleshooting, helping customers optimize adjustments and ensure smooth method transitions.
Our Product Recommendations
Empower™ Software
Designed for regulatory confidence in modern pharmaceutical testing workflows
Empower Data Viewer
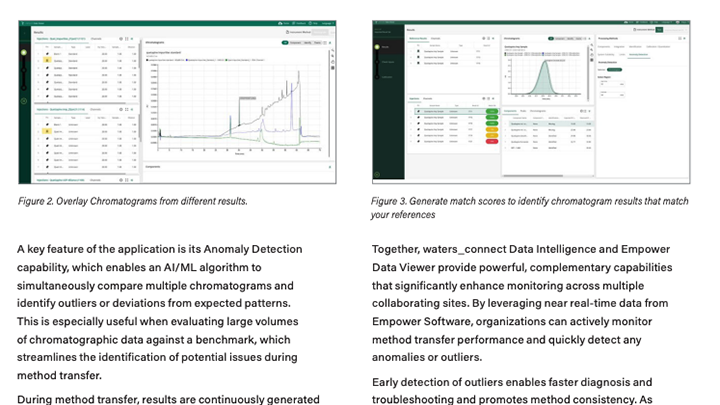
Data sharing and collaboration cloud application for near real time monitoring
waters_connect Data Intelligence
Access deeper insights, improve laboratory operations and increase audit readiness
Tools To Support Method Adjustment
The Intelligent Method Transfer App (iMTA)
The iMTA is a specialized application developed by Waters to streamline and simplify the process of transferring LC methods between different HPLC systems. It is designed to migrate methods from legacy or third-party systems to the Waters Alliance iS HPLC System.
What exactly does the iMTA do?
- Automated Method Translation: iMTA translates instrument methods from Empower projects or other supported systems into compatible formats for the receiving LC platform. This eliminates manual transcription errors and saves significant time.
- System-Aware Adjustments: It accounts for differences in system design—such as dwell volume, pump configuration, and mixer size—ensuring that transferred methods maintain their original chromatographic performance.
- Error Reduction: By automating the translation process, iMTA reduces the risk of human error and ensures consistency across labs and instruments.
- Time Efficiency: It bypasses the need for extensive re-optimization, allowing analysts to focus on data quality and interpretation rather than method redevelopment.
Video: Find out how the iMTA brings benefit
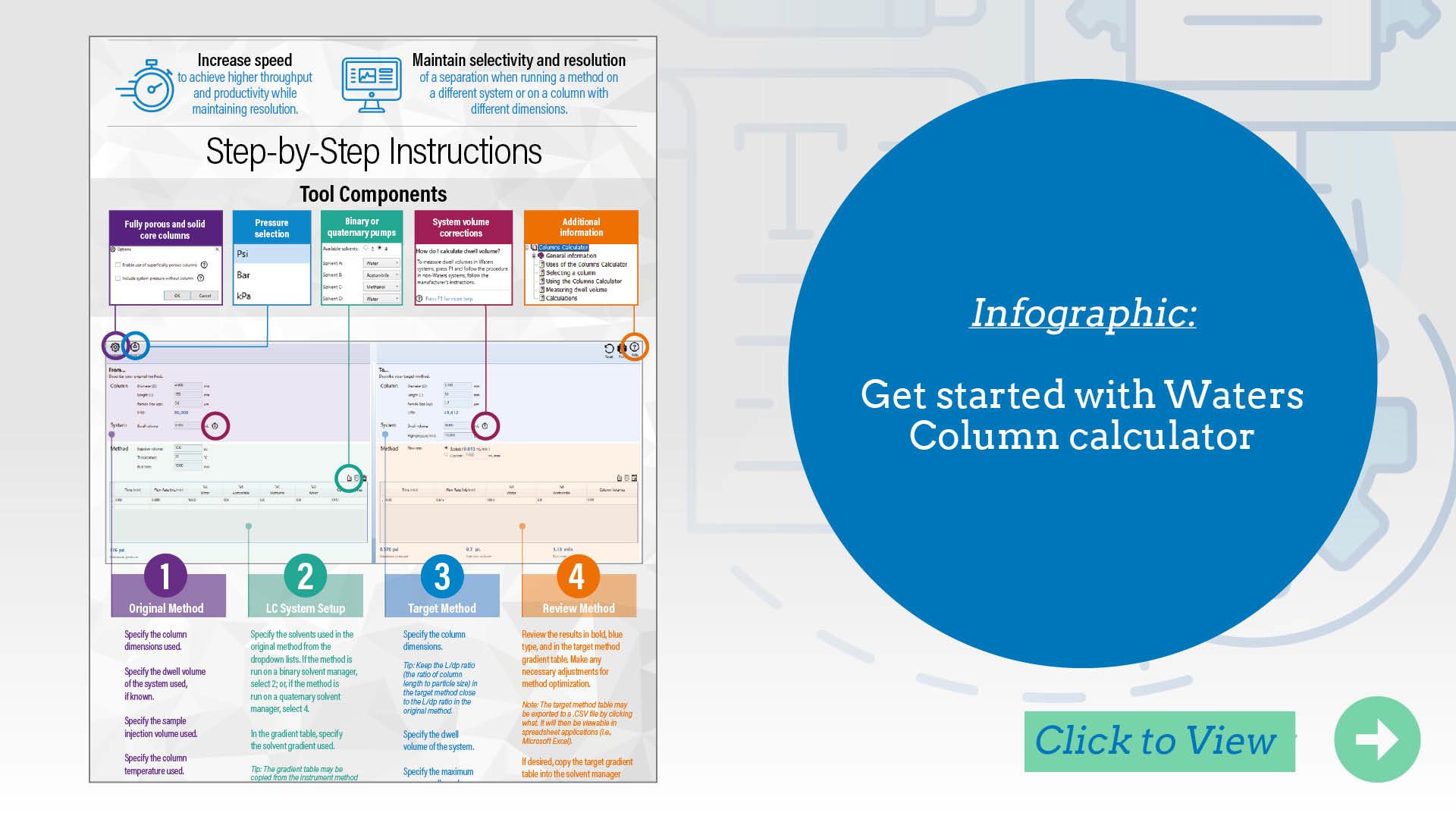
Waters Column Calculator: Simplifies LC column scaling and selection.
The Waters Column Calculator simplifies method scaling across different column dimensions and particle sizes. It automatically adjusts key parameters such as flow rate, injection volume, and gradient time based on the specifications of both the original and target columns. The tool also recommends pre- or post-injection volumes for Gradient SmartStart, helping compensate for dwell volume differences between systems. With its latest update, the Column Calculator now also indicates whether the L/dp ratio (column length divided by particle diameter) of the new column falls within USP <621> guidelines, helping users stay compliant with regulatory expectations during method adjustment.
Use it to save time, reduce manual errors, and confidently transfer methods across platforms.
Explore Waters Column Chemistries
Gradient SmartStart: Aligns gradients, simplifies method transfer workflows
When translating analytical methods between HPLC systems, compensating for differences in dwell volume, gradient delay, and pump design is vital to preserving retention time and separation performance. Such variations can greatly affect reproducibility, particularly in methods with shallow gradients or sensitive analytes. Waters’ Gradient SmartStart technology helps by adjusting the gradient start time relative to injection, ensuring consistent chromatographic behavior across systems, including those from other vendors. By aligning retention profiles and reducing variability, Gradient SmartStart streamlines method transfer, minimizes re-optimization, and strengthens data integrity—critical in regulated and cross-platform workflows.
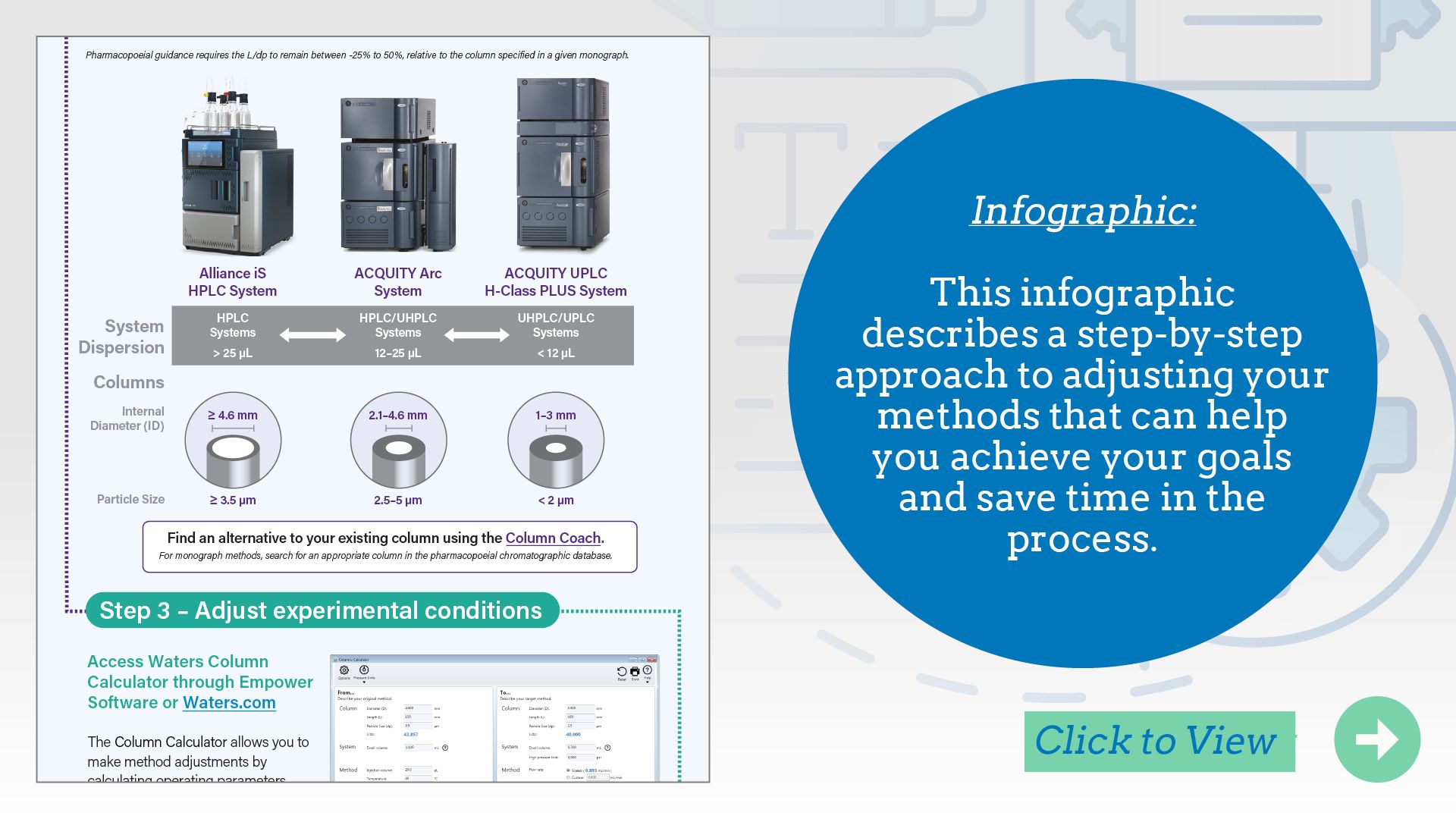
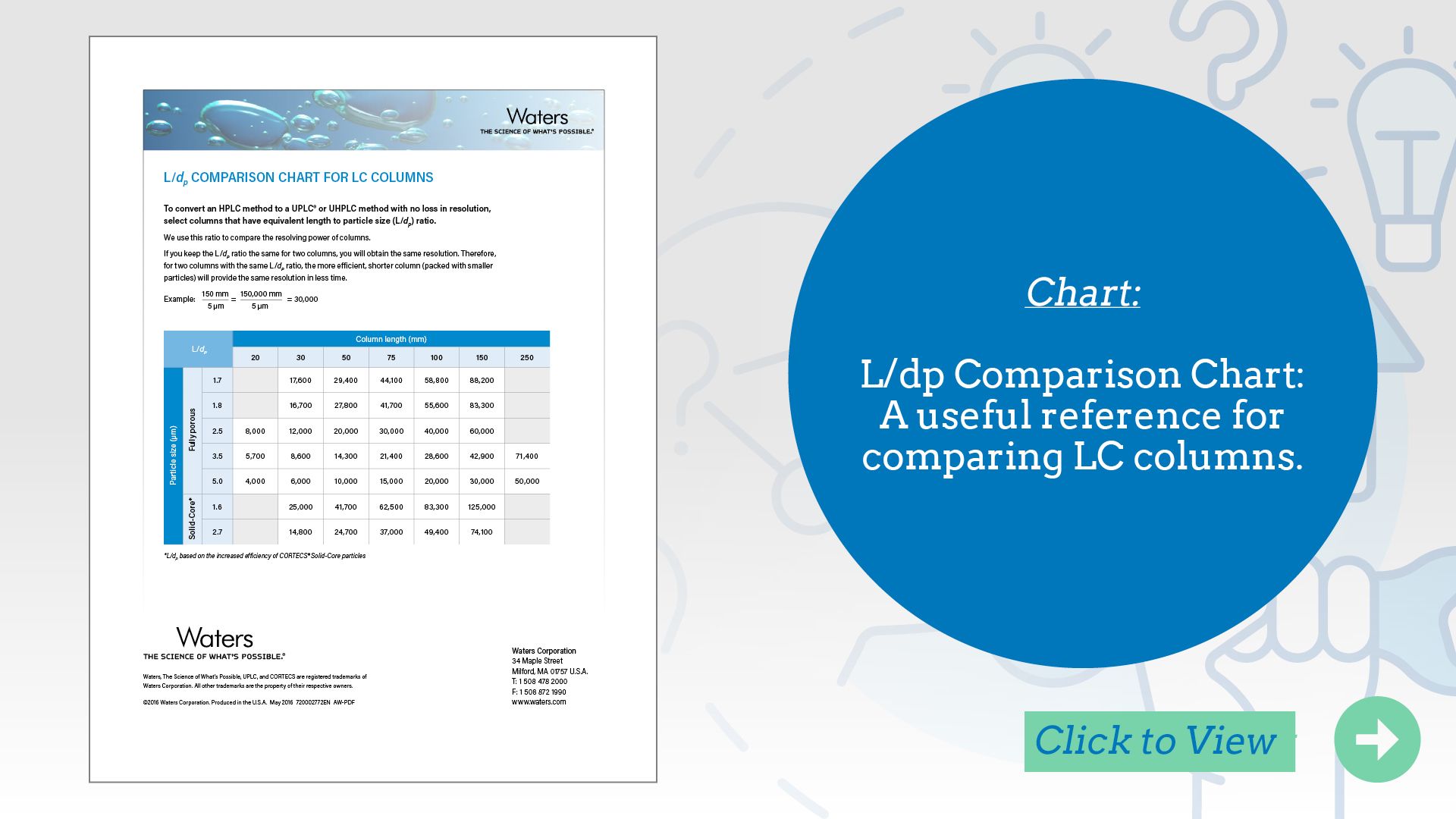
L/dp Comparison Chart: A useful reference for comparing LC columns.
When translating LC methods across different column formats, understanding the impact of column geometry is essential to maintain chromatographic performance. Waters’ L/dp Comparison Chart provides a practical solution by helping scientists compare columns based on the ratio of column length (L) to particle diameter (dp)—a key factor influencing separation efficiency and resolution. This tool simplifies method scaling and optimization by allowing users to select equivalent or alternative columns that preserve analytical performance. It’s especially valuable during method transfer, modernization, or troubleshooting, where minimizing variability and maintaining consistency are critical. By using the chart, analysts can make informed decisions that support robust, reproducible workflows across platforms and applications.